
The pharmaceutical R&D sector of Leadingpharm has established four research institute bases in Beijing, Zhengzhou, Chongqing and Hangzhou. Each research institute has its own characteristics, and its research directions are different in combination with local advantages and clinical needs.
-
1000Products
More than 1000 drugs have been developed
-
500People
More than 500 professional teams in pharmaceutical R&D section
-
19years
19 years of pharmaceutical research experience in applying for drug registration
The scope of services includes:
-
01Study on Synthesis Process of API and Excipients
-
02Research on formulation and technology
-
03Drug quality research and method verification
-
04Impurity customization
-
05Substance detection
-
06Research on special impurities
-
07Compatibility Study
-
08Registration risk assessment and application data writing
Architecture of the pharmaceutical R&D platform
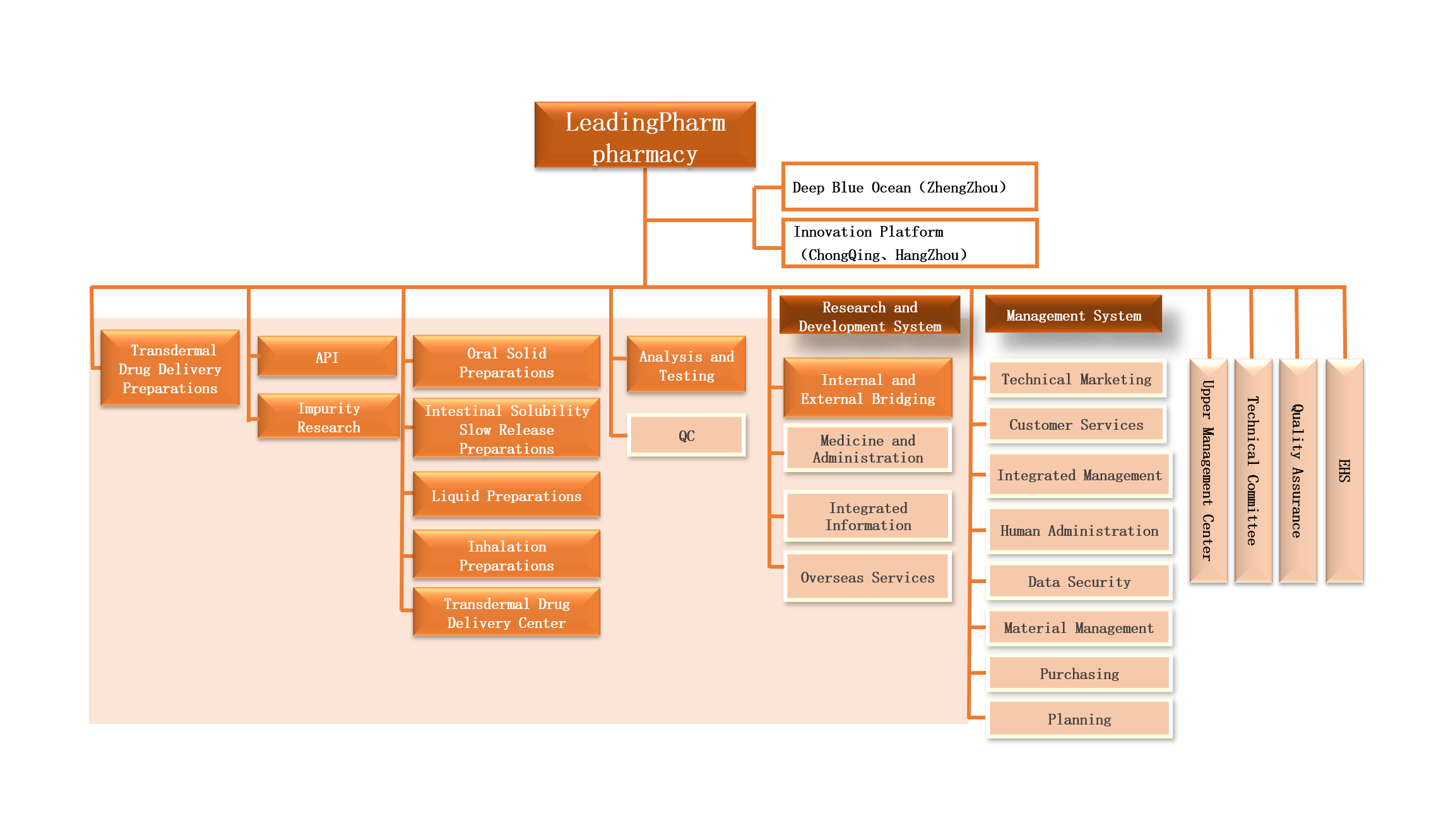
We has nearly 20 years of experience in the field of API synthesis and preparation R&D, and can provide high-quality API/excipient synthesis process development and impurity customization services, formulation and process development, covers conventional oral drug delivery platforms and high-end drug delivery platforms such as blood brain barrier drug delivery, oral nasal aerosol drug delivery, controlled release drug delivery to skin and mucosa, targeted drug delivery, etc. A strong R&D team conducts project management in the form of a business unit, emphasizing "professional people focus on professional things", and specially employs well-known experts at home and abroad to form a technical committee to effectively solve project bottlenecks and problems and promote the project to be carried out as planned, can support innovative drug enterprises, MAH holders and generic drug R&D companies to carry out innovative drug/improved new drug R&D, generic drug consistency evaluation and R&D application.
Pharmaceutical R&D Project Management Mode
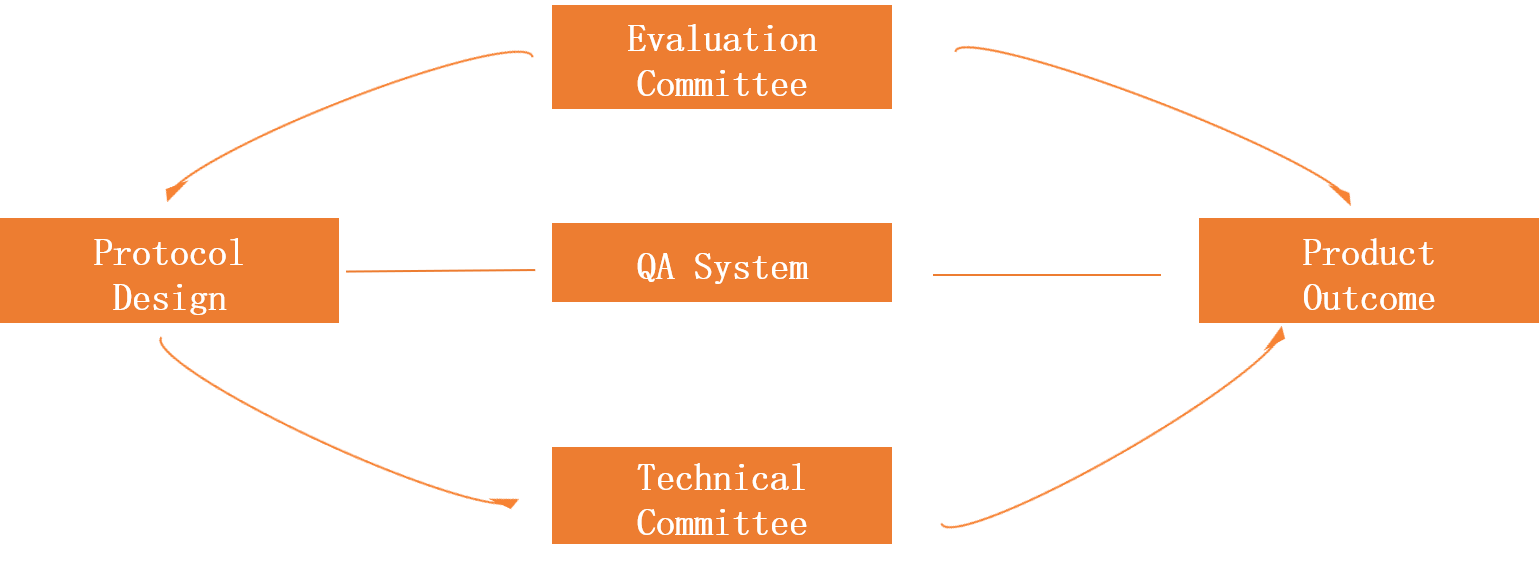 ① Conference Management Center: according to the characteristics of the project, experts from inside and outside of the company will set up an expert group to check each key node of the project based on the guiding principles and the company's standards, strengthen the whole process control of the project to ensure the smooth application of the project,strengthen the whole process control of the project to ensure the smooth application of the project.
① Conference Management Center: according to the characteristics of the project, experts from inside and outside of the company will set up an expert group to check each key node of the project based on the guiding principles and the company's standards, strengthen the whole process control of the project to ensure the smooth application of the project,strengthen the whole process control of the project to ensure the smooth application of the project.
②Technical Committee: Organize and solve technical problems. In response to important technical problems encountered in the project development process, the technical committee organized a team of experts from the company to provide technical support to solve technical problems and ensure the smooth development of the project.
③ QA:In the whole process of project research and development, conduct normative inspection of the site and documents to ensure the reliability and standardization of experimental data and the high-quality pass of on-site inspections.

 Hot line:010-61006450
Hot line:010-61006450
 EN
EN





 010-61006450
010-61006450 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
